Cardiogenic Shock from Severe Right Ventricular Endothelial Dysfunction and Raynaud’s with Digital Ischemia in a Patient with Newly Diagnosed Systemic Lupus Erythematous/Mixed Connective Tissue Disease
From Grand Rounds from HSS: Management of Complex Cases | Volume 14, Issue 3
Case Report
A 32-year-old woman with well-controlled asthma and depression was hospitalized with right ventricular (RV) failure and pulmonary hypertension crisis.
One year earlier, she had developed discoloration of the fingers with wounds, which she attributed to occupational injury while handling boxes. She experienced unintentional weight loss and fatigue and in the 2 weeks before admission developed puffy fingers, joint pain, rash, digital ulcers consistent with severe Raynaud’s phenomenon (Figure 1). The patient presented to the emergency department after 2 syncopal episodes and onset of chest pain radiating to the left arm.
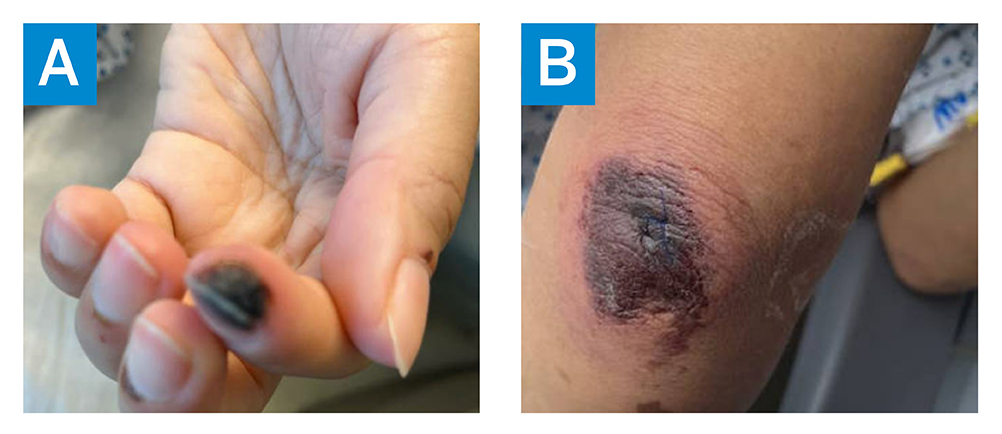
Figure 1: The patient’s fingers 2 weeks before hospitalization showing (A) Raynaud’s phenomenon, with digital ischemia, and (B) retiform purpura.
On presentation her temperature was 36.8°C, blood pressure was 102/74 mmHg, heart rate was 108 beats per minute, and oxygen saturation was 99%. She was noted on examination to have puffy hands, digital ulcers with dry gangrene, periungual erythema with normal nailfold capillaroscopy, ankle synovitis, and retiform purpura over the chest and both arms. Laboratory results showed elevated brain natriuretic peptide (7508 pg/mL), lactate (3.7 mmol/L), and sensitivity troponin T (21 ng/dL), plus mild proteinuria (urine protein-creatinine ratio, 0.35 g/g). An echocardiogram (Figure 2) demonstrated severe RV dilatation with severely reduced systolic function, severe tricuspid regurgitation, and small pericardial effusion; left ventricular ejection fraction was normal. The estimated pulmonary arterial systolic pressure was 55 to 60 mmHg, consistent with moderate pulmonary arterial hypertension (PAH). Computed tomography of the chest demonstrated interlobar septal thickening and multifocal lymphadenopathy.
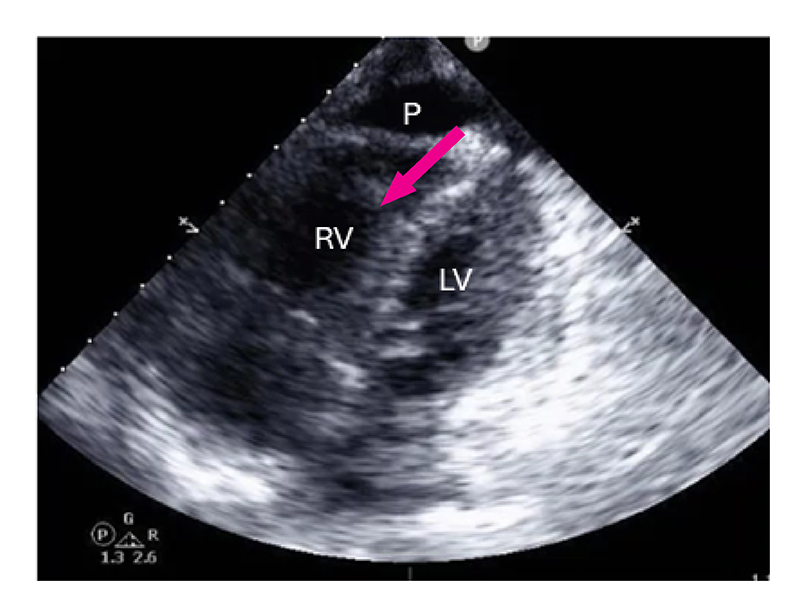
Figure 2: 4-chamber view echocardiography, with findings consistent with moderate pulmonary arterial hypertension.
LV: left ventricle, RV: right ventricle, P: pericardial effusion.
Right heart catheterization showed moderate PAH with pulmonary arterial pressure of 46/24 mmHg (mean 32 mmHg) and normal wedge pressure, suggestive of RV dysfunction disproportionate to PAH (Table 1). Rheumatologic serologies were significant for anti-nuclear antibody (1:1280), positive double-stranded DNA (73 IU),anti-Smith antibody (187 AU/mL), and ribonucleoprotein (128 U), as well as anti-Ro/SSA, anti-La/SSB, rheumatoid factor, and histone antibodies. Results were also notable for C3 and C4 hypocomplementemia, positive direct antiglobulin testing for immunoglobulin G, and negative antiphospholipid syndrome antibodies.
Table 1. Right heart catheterization results, pre- and post-dobutamine administration.
| RA (mmHg) | PA (mmHg) | PCWP (mmHg) | CI (L/min/m2) | PVR (Wood units) | |
| Post-dobutamine RHC | 2 | 58/14 (mean: 35) | 8 | 3.2 | 6.3 |
| Pre-dobutamine RHC | 5 | 46/24 (mean: 32) | 10 | 1.7 | 8.3 |
A diagnosis was made of overlap connective tissue disease with features of systemic lupus erythematosus (SLE) and mixed connective tissue disease (MCTD) [1]. She was started on methylprednisolone 1 mg/kg IV to treat pericarditis and inflammatory arthritis and on dobutamine and sildenafil for PAH and RV failure. She was transferred to the cardiac intensive care unit.
Ambrisentan was initiated but stopped due to worsening hypoxia secondary to pulmonary edema. She was started on hydroxychloroquine and mycophenolate mofetil. She developed worsening chest pain with steroid taper and was treated with methylprednisolone 250 mg for 3 days, with improvement. Subsequent right heart catheterization while on dobutamine showed stable, moderate PAH. Because the RV dysfunction was out of proportion to her PAH, additional studies were undertaken. Cardiac magnetic resonance imaging showed elevated myocardial extracellular volume fraction.
Endomyocardial biopsy showed mild diffuse interstitial fibrosis and arteriolar intimal hyperplasia, with no evidence of infiltrative cardiomyopathy. There was focal microvascular sclerosing involving interstitial capillaries, which was suggestive of repeated immune-mediated endothelial injury (Figure 3A). On immunofluorescence, there was positive microvascular staining of complement components including C4d, which was suggestive of local complement activation of the classical pathway (Figure 3B).
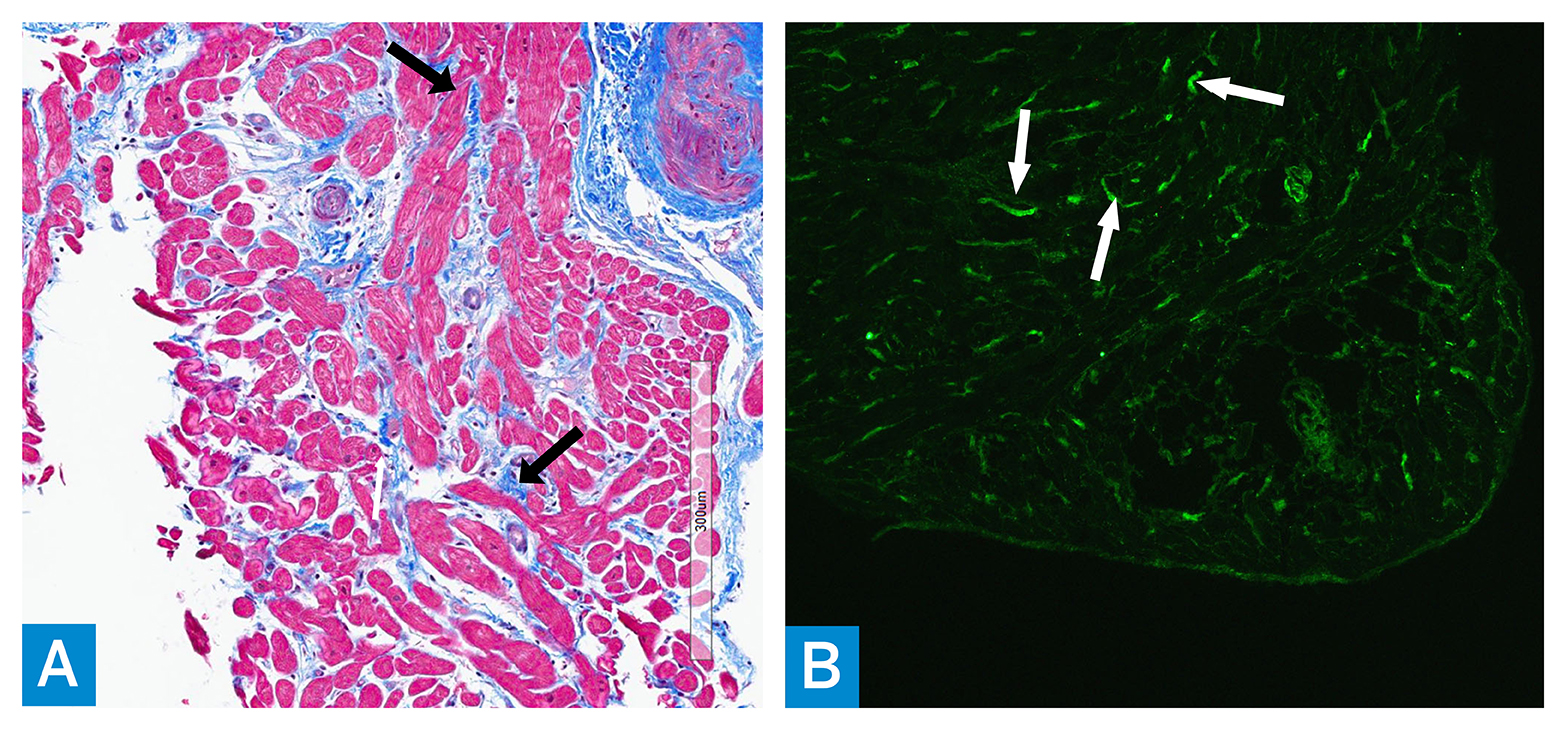
Figure 3: (A) Trichrome stained myocardial biopsy tissue shows increased bundles of collagen (blue) (arrows) in between the myocytes (red); magnification ×200. (B) Immunofluorescence microscopy using antibody to complement component C4d shows diffuse, linear, positive staining mainly within the interstitial capillary walls (arrows); magnification ×100.
She was treated with rituximab for connective tissue disease–associated myocardial dysfunction in the setting of vasculopathy and antibody-mediated classical complement pathway activation. During a complex hospitalization, escalating doses of vasodilator therapy were needed to manage severe Raynaud’s. After 1 month of hospitalization, dobutamine was stopped and the patient was discharged on a steroid taper. Following immunosuppression, vasodilator therapy, and heart failure–directed treatment, her left and right ventricular size/function were both normal, with no echocardiographic evidence of pulmonary hypertension.
In this episode of HSS Presents, author Dr. Genna Braverman talks with cardiology and rheumatology colleagues about the complex interplay between the heart and rheumatic diseases.
Discussion
We describe a rare case of endothelial dysfunction affecting the right ventricle leading to cardiogenic shock in the setting of SLE/MCTD, treated successfully with advanced therapies in combination with immunosuppression, including B-cell depleting therapy. The patient’s myocardial dysfunction was thought to be mediated by vasculopathy in the setting of antibody-mediated complement activation. Such cardiac endothelial dysfunction resembles that seen in systemic sclerosis and SLE [2, 3]. However, cases of RV failure from these diseases typically result from PAH secondary to endothelial dysfunction rather than from selective targeting of the right ventricular endothelium [4].
References
- Moschetti L, Piantoni S, Vizzardi E, et al. Endothelial dysfunction in systemic lupus erythematosus and systemic sclerosis: a common trigger for different microvascular diseases. Front Med (Lausanne). 2022 8;9:849086. doi: 10.3389/fmed.2022.849086.
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des. 2014;20(4):536-44. doi: 10.2174/13816128113199990555. PMID: 23565639.
- Guo L, Li M, Chen Y, et al. Anti-endothelin receptor type a autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertension. Arthritis Rheumatol. 2015;67(9):2394-402. doi: 10.1002/art.39212.
- Kato M, Sugimoto A, Atsumi T. Diagnostic and prognostic markers and treatment of connective tissue disease-associated pulmonary arterial hypertension: current recommendations and recent advances. Expert Rev Clin Immunol. 2020;16(10):993-1004. doi: 10.1080/1744666X.2021.1825940.

